Engineering Medicine with Organ-Chips
a fast-track platform for translational research
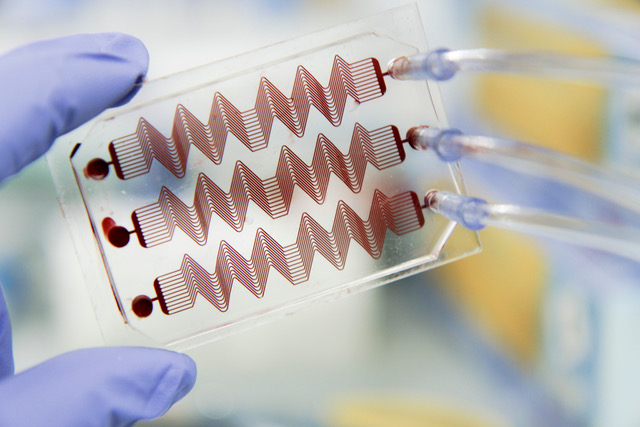
Animals have long been the bedrock behind our understanding of human physiology and drug discovery despite the awareness of the discordance between animal and human studies. In past three decades, the number of FDA-approved drugs per billion US dollars spent on R&D has actually decreased monotonically. Our research aspires to reverse this poor trend by innovating new bioengineered approaches, paradigms and tools that make a positive impact to medicine and healthcare economics. Our lab is achieving this ambition by harnessing the basic knowledge and methods offered by cell and molecular biology, biomechanics, microfabrication technology, biomaterials, and mathematics; and reconstructing the physical microenvironment of human tissues and organs in microfluidic devices, also known as organs-on-chip. Our lab particularly specializes in making patient-specific organ-chip models of rare and orphan diseases with an emphasis on vascular and hematological diseases and its long-term manifestations in cancer, diabetes and infections. By establishing and leveraging extensive collaborations across the Texas Medical Center, our group has made contributions in advancing the fundamental understanding and drug discovery of sickle cell disease, type-1 diabetes, vein thrombosis, ovarian cancer, lymphedema and COVID-19.
Funding








Current Directions
Vein-Chip: Discovery of pathophysiology & treatments of chronic venous insufficiency and thrombosis
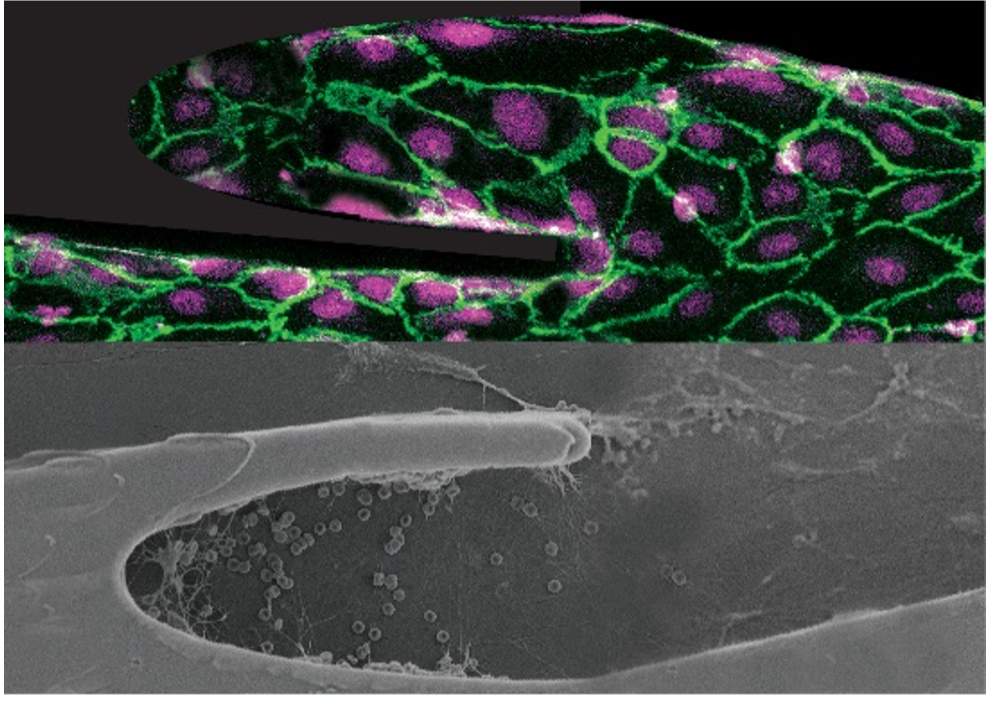
Vascular Organ-Chips made from blood: Enabling personalized medicine of sickle cell disease
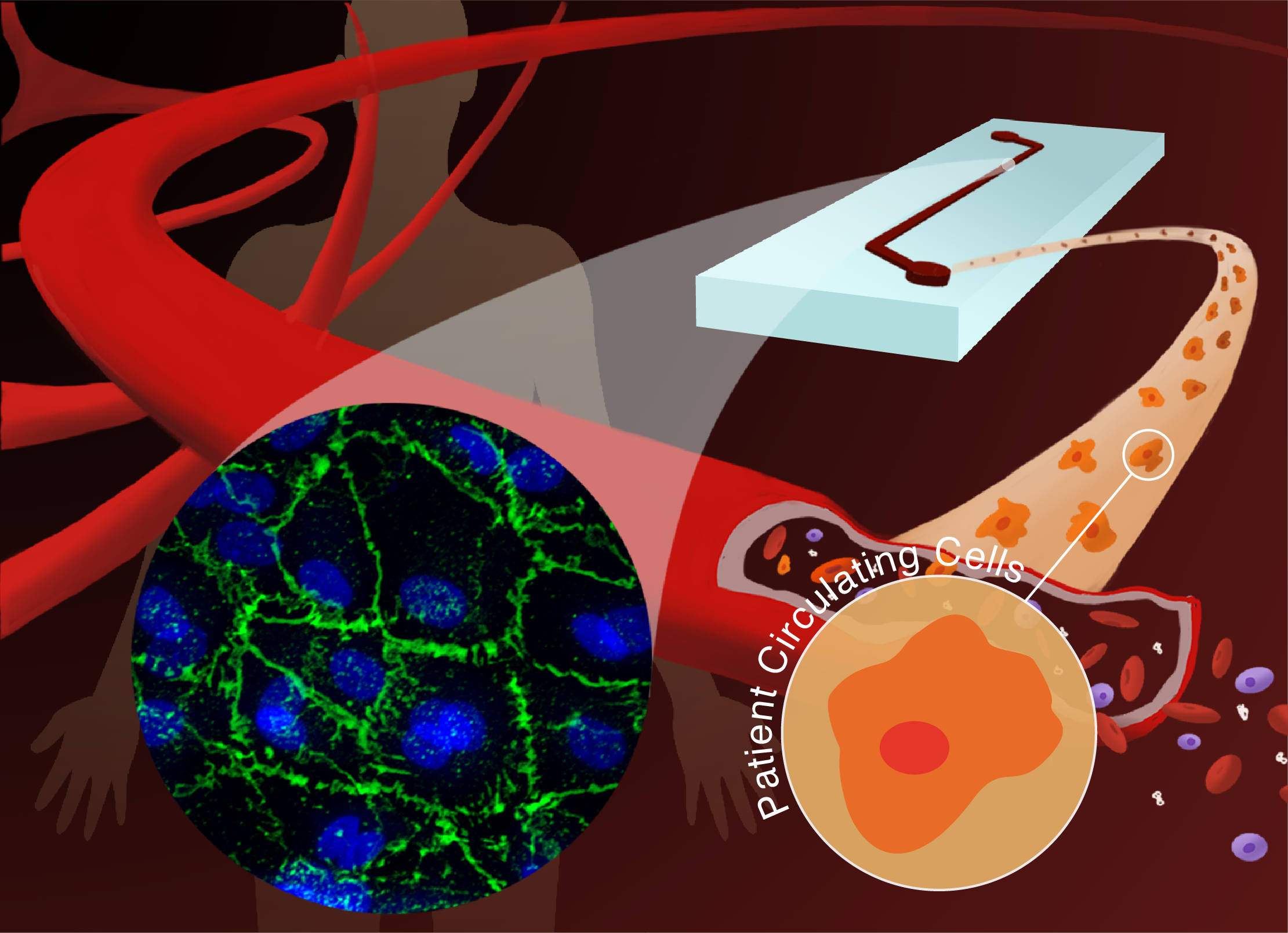
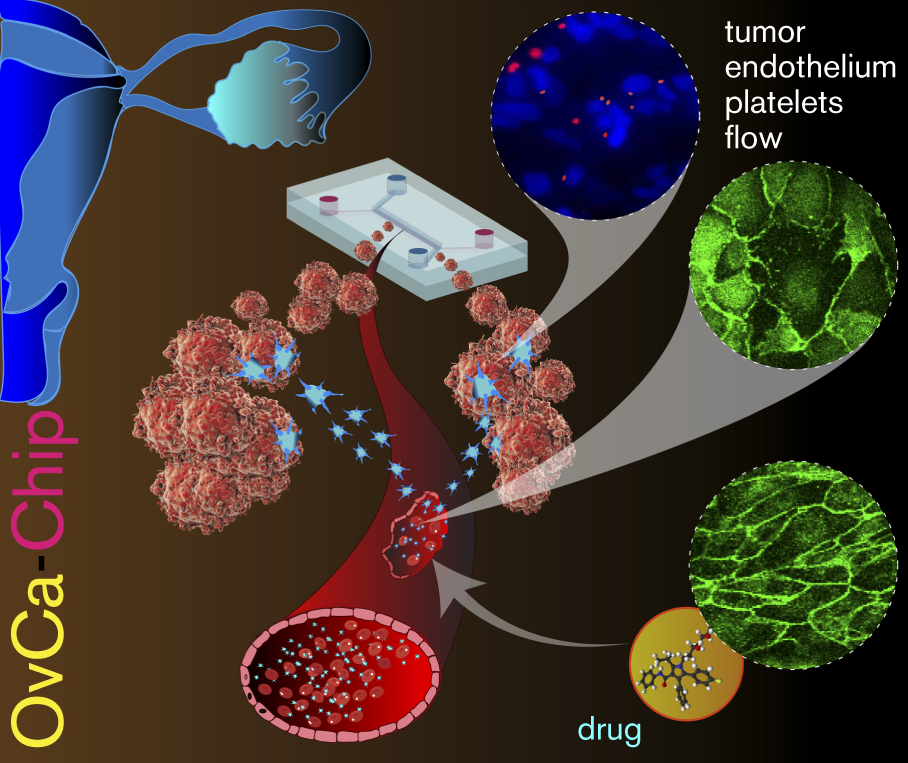
Tumor Microenvironment-Chip: Discovery of platelet immunopathology & combinatorial therapy in cancer
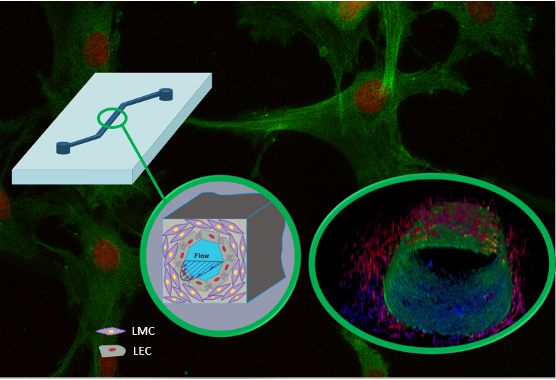
Multicellular Vessel-Chip: Recapitulate hydrodynamics and mechanobiology of lymphedema and atherosclerosis
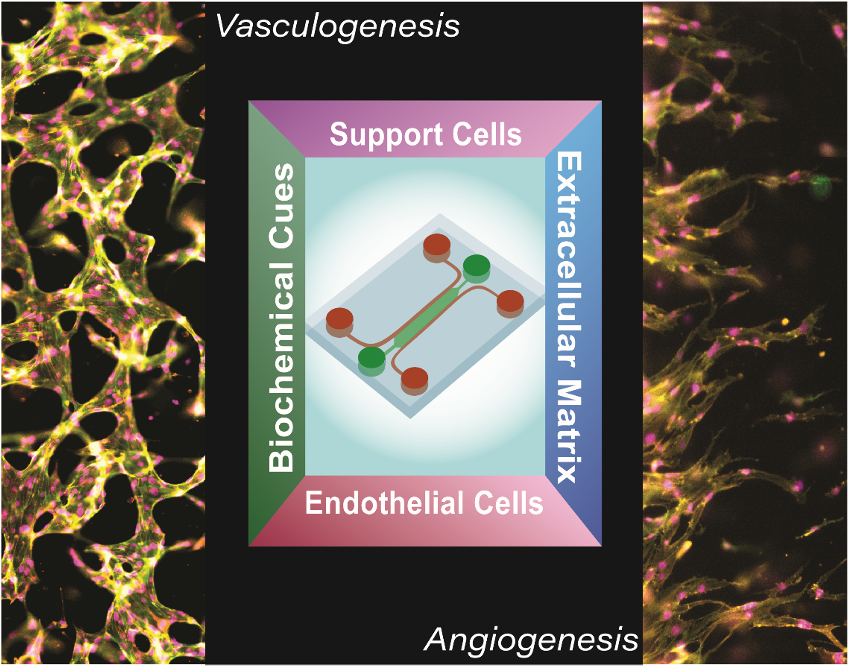
Vascularized Organ-Chips
Microfluidic Devices of Coagulation and Platelet Function